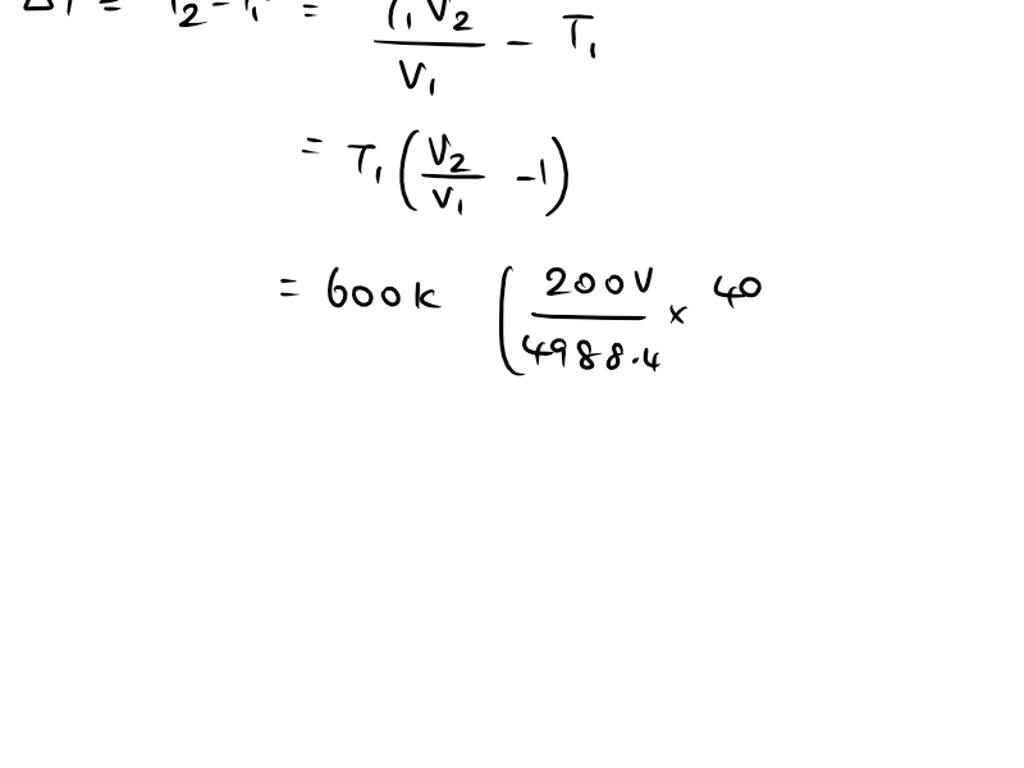Specific Heat Of Nitrogen At Constant Volume . 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. specific heat for an ideal gas at constant pressure and volume. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. The heat capacity at constant volume of nr = 1 j·k −1 of any. specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. I sochoric specific heat (c v) is. When a given amount of heat is added to different substances, their temperatures increase by. The phase diagram of nitrogen is shown below the table.
from www.numerade.com
the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. I sochoric specific heat (c v) is. specific heat of nitrogen is 1.04 j/g k. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. When a given amount of heat is added to different substances, their temperatures increase by. specific heat for an ideal gas at constant pressure and volume. The heat capacity at constant volume of nr = 1 j·k −1 of any. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat.
SOLVED Nitrogen is isobarically expanded from 100 kPa and 600 K until
Specific Heat Of Nitrogen At Constant Volume The phase diagram of nitrogen is shown below the table. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. When a given amount of heat is added to different substances, their temperatures increase by. I sochoric specific heat (c v) is. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. The phase diagram of nitrogen is shown below the table. The heat capacity at constant volume of nr = 1 j·k −1 of any. specific heat for an ideal gas at constant pressure and volume. specific heat of nitrogen is 1.04 j/g k. 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket.
From www.toppr.com
If CP and CV denote the specific heats of nitrogen per unit mass at Specific Heat Of Nitrogen At Constant Volume 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. specific heat of nitrogen is 1.04 j/g k. The heat capacity at constant volume of nr = 1 j·k. Specific Heat Of Nitrogen At Constant Volume.
From www.chegg.com
Solved Problem 3 A pistoncylinder device contains 1.2 kg Specific Heat Of Nitrogen At Constant Volume specific heat of nitrogen is 1.04 j/g k. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. specific heat for an ideal gas at constant pressure and volume. 53 rows. Specific Heat Of Nitrogen At Constant Volume.
From www.doubtnut.com
Calculate the difference between two specific heats of 1 g of nitrogen Specific Heat Of Nitrogen At Constant Volume the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. specific heat for. Specific Heat Of Nitrogen At Constant Volume.
From www.toppr.com
If CP and CV denote the specific heats of nitrogen per unit mass at Specific Heat Of Nitrogen At Constant Volume the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. specific heat for an ideal gas at constant pressure and volume. I sochoric specific. Specific Heat Of Nitrogen At Constant Volume.
From www.grc.nasa.gov
Specific Heats Specific Heat Of Nitrogen At Constant Volume the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. specific heat for an ideal gas at constant pressure and volume. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. I sochoric specific heat (c v) is. the specific heat capacity. Specific Heat Of Nitrogen At Constant Volume.
From www.toppr.com
If C p and C v denote the specific heats of nitrogen per unit mass at Specific Heat Of Nitrogen At Constant Volume I sochoric specific heat (c v) is. specific heat of nitrogen is 1.04 j/g k. specific heat for an ideal gas at constant pressure and volume. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. The heat capacity at constant volume of nr = 1 j·k −1. Specific Heat Of Nitrogen At Constant Volume.
From www.numerade.com
Find the specific heat at constant volume of 1.00 cm^3 of radlation in Specific Heat Of Nitrogen At Constant Volume specific heat of nitrogen is 1.04 j/g k. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. I sochoric specific heat (c v) is.. Specific Heat Of Nitrogen At Constant Volume.
From www.chegg.com
Solved Part A Compute the specific heat capacity at constant Specific Heat Of Nitrogen At Constant Volume 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. When a given amount of heat is added to different substances, their temperatures increase by. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. . Specific Heat Of Nitrogen At Constant Volume.
From www.toppr.com
Cp and Cy are specific heats constant pressure and constant volume Specific Heat Of Nitrogen At Constant Volume 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. specific heat of nitrogen is 1.04 j/g k. specific heat for an ideal gas at constant pressure and volume. The phase diagram of nitrogen is shown below the table. 55 rows the table of specific heat capacities. Specific Heat Of Nitrogen At Constant Volume.
From www.chegg.com
The equation of state of gaseous nitrogen at low Specific Heat Of Nitrogen At Constant Volume 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. I sochoric specific heat (c v) is. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. The phase diagram of nitrogen is shown below the table. the specific heat (= specific heat capacity). Specific Heat Of Nitrogen At Constant Volume.
From www.toppr.com
Cp and Cv are specific heats at constant pressure and constant volume Specific Heat Of Nitrogen At Constant Volume When a given amount of heat is added to different substances, their temperatures increase by. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. I sochoric specific heat (c v) is. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is. Specific Heat Of Nitrogen At Constant Volume.
From www.numerade.com
SOLVED Nitrogen is isobarically expanded from 100 kPa and 600 K until Specific Heat Of Nitrogen At Constant Volume When a given amount of heat is added to different substances, their temperatures increase by. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. specific heat of nitrogen is 1.04 j/g k. The. Specific Heat Of Nitrogen At Constant Volume.
From www.toppr.com
If C p and C v denote the specific heats of nitrogen per unit mass at Specific Heat Of Nitrogen At Constant Volume under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. I sochoric specific heat (c v) is. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as. Specific Heat Of Nitrogen At Constant Volume.
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of Specific Heat Of Nitrogen At Constant Volume under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 53 rows isobaric specific heat (c p) is used for substances in a constant pressure (δp = 0) system. The heat capacity at constant volume of nr = 1 j·k −1 of any. specific heat of nitrogen is 1.04 j/g k. specific. Specific Heat Of Nitrogen At Constant Volume.
From www.numerade.com
SOLVED Nitrogen enters a steadyflow heat exchanger at 150 kPa, 10°C Specific Heat Of Nitrogen At Constant Volume The phase diagram of nitrogen is shown below the table. The heat capacity at constant volume of nr = 1 j·k −1 of any. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and. Specific Heat Of Nitrogen At Constant Volume.
From askfilo.com
60. Find out the molar specific heat of Nitrogen at constant volume if fo.. Specific Heat Of Nitrogen At Constant Volume specific heat for an ideal gas at constant pressure and volume. The heat capacity at constant volume of nr = 1 j·k −1 of any. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. the specific heat capacity of a substance, especially a gas, may be significantly. Specific Heat Of Nitrogen At Constant Volume.
From www.youtube.com
The difference between the principal specific heats of nitrogen is 300 Specific Heat Of Nitrogen At Constant Volume specific heat for an ideal gas at constant pressure and volume. the specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is. When a given amount of heat is added to different substances, their temperatures increase by. I sochoric specific heat (c v) is. The heat. Specific Heat Of Nitrogen At Constant Volume.
From www.toppr.com
CP and CV are specific heats at constant pressure and constant volume Specific Heat Of Nitrogen At Constant Volume I sochoric specific heat (c v) is. The phase diagram of nitrogen is shown below the table. under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. specific heat for an ideal gas at constant pressure and volume. When a given amount of heat is added to different substances, their temperatures increase by. . Specific Heat Of Nitrogen At Constant Volume.